Fullerene
Fullerenes – one of the most outstanding achievements of the late 20th century in chemistry, is an allotropic modification of carbon.
Showing all 3 results
-
Fullerene C60
55 € – 5,060 € Select options -
Fullerene C70
203 € – 8,970 € Select options -
Fullerenol
92 € – 23,460 € Select options
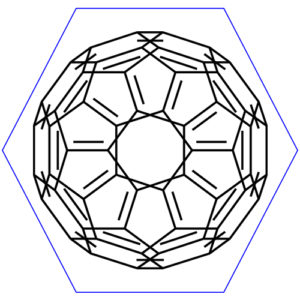
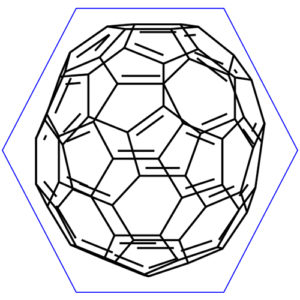
The fullerene molecule is a spheroidal hollow framework molecule of an even number of covalently bonded carbon atoms located at the vertices of hexagons or pentagons.
Inside the molecule, there is a cavity into which atoms and molecules of other substances can be introduced. In their own way, Fullerenes are similar in structure to aromatic compounds. Fullerenes usually available as:
- A mixture of fullerenes (a mixture of fullerenes C60 + C70 in a ratio of 70% C60 and 30% C70)
- Fullerene C60 (Nanopowder (crystals) of fullerene C60 of various purities from 99%)
- Fullerene C70 (Nanopowder (crystals) of fullerene C70 of various purities from 98%)
- Fullerenol (Water-soluble derivatives of fullerene containing hydroxy-OH groups from 12 to 24, – C60 (OH) 24,
- C70 (OH) 12, carboxylated fullerene C60 (C (COOH) 2) 3)
- Functionalized fullerenes (Water-soluble derivatives of fullerene with grafted amino acids and polymers (C60-Gly2 C60-Lys2 C60-Met2 C60-Arg8 C60-Thr3 C70-Thr PCBM (C60))
- Endohedral fullerenes (Fullerenes in the cavity of a molecule of which one or more atoms, for example, metals, are placed)
As cliché as it may sound, there is absolutely no doubt that the discovery of C60 fullerene was a major breakthrough. And you are going to learn what makes it so unique.
This article will provide you with all important facts you need to know in order to understand its huge potential. You will learn about its discovery, definition, chemical and physical properties and also about its applications.
Nevertheless, we are not going to stick to the plain information only. You will also get an insight into the exciting work of the scientists, who discovered this amazing molecule.
And in addition you will get to know other types of fullerenes as well.
C60 Fullerene – The Beginning of Nanotechnology
The first one who actually started a concept of nanotechnology was a famous physicist Richard Feynman. It was discussed in his talk ‘There’s Plenty of Room at the Bottom’. In this talk he assumed there is a possibility to synthesize via direct manipulation of atom.
But the first one who actually used the term Nano-technology was Japanese professor at Tokyo University of Science, Norio Taniguchi. That was in 1974.
Few years later thanks to the invention of the scanning tunneling microscope (1981), it was possible to visualize the individual atoms and bonds. That was the first of the two major breakthroughs that led to the popularization and growth of nanotechnology. The second one was the unexpected discovery of fullerenes (1985), specifically the discovery of C60 molecule.
So What Are Fullerenes?
Let’s start with the basic definition. Fullerenes are in fact allotropes of carbon. They can form a hollow sphere, ellipsoid, tube and many other shapes.
- Spherical fullerenes – C60 molecule also known as Buckminsterfullerene or buckyballs.
- Cylindrical fullerenes – carbon nanotubes also known as buckytubes
Fullerene could be compared by its structure to graphite. It consists of piled up graphene sheets of connected hexagonal rings. If they are cylindrical they have to contain pentagonal rings as well.
Types of Fullerenes
- Buckyballs structure
- Nanotubes
- Megatubes
- Polymers
- Nano “onions”
- Linked “ball-and-chain” dimers
- Fullerene rin
This article is devoted to the member of the first category, molecule C60. However, we will get back to the other types of fullerenes later as well.
An Astonishing Discovery – What Is C60 Buckminsterfullerene?
You already know this molecule was the first discovered fullerene. It consists of sixty carbon atoms. If you look at its structure it resembles the soccer (football) ball which is made of twenty hexagons and twelve pentagons, where the hexagons represent the white parts and pentagons black ones. The structure of buckyballs is cage-like ring fused. To be precise it is a truncated icosahedron. There is carbon atom at each vertex of each polygon. The bond is along each polygon edge.
Properties
| Chemical Formula | C60 |
| Molar Mass | 720.66 g∙mol¯¹ |
| Appearance | Dark needle-like crystals |
| Density | 1.65 g/cm³ |
| Melting Point | Sublimates at ~ 600 °C (1,112 °F; 873 K) |
| Solubility in Water | Insoluble in water |
Structure
| Crystal structure | Face-centered cubic, cF1924 |
| Space Group | Fm3m, No. 225 |
| Lattice Constant | a = 1.4154 nm |
As it was mentioned before its discovery was a major breakthrough that set the base of a new era. Some even predict that the nanotechnology will have even bigger impact on our lives than the electronics revolution.
It was also recognized by the Royal Swedish Academy of Sciences. In 1996, the trio scientists who discovered fullerenes were awarded the Nobel Prize in Chemistry.
Who Discovered Molecule C60?
The initiator of the experiments which took place at the Rice University in Houston, Texas, was Sir Harold Walter Kroto.
Harry Kroto was an English chemist. His interest was in astrochemistry. He wanted to study the origins of the long linear carbon chains molecule. The problem was that he did not have the right equipment for such a purpose.
However, he was not going to give up that easily. In 1984 he flew to Texas to attend a conference, where he met his friend Robert Curl, who happened to be a colleague of Richard Smalley. Both of them worked together at the Rice University.
Harry Kroto knew that Richard Smalley constructed a special device AP2. This instrument let the scientists to investigate clusters of any element. Naturally, Kroto wanted to use this machine for his experiments.
However, Richard Smalley did not agree to borrow him AP2 immediately. He had some experiments of his own which he wanted to finish, so Harry had to wait. In fact he had been waiting the whole year. In 1985 Smalley finally agreed and Kroto flew right away to the USA.
That is how the famous trio teamed up. Their research took place in the labs at the Rice University. At last Kroto, Smalley and Curl with the assistance of James R. Heath and Sean O’Brian started their experiments.
What was the actual result of these experiments?
- As Kroto assumed, they found the long carbon chains.
- They observed something completely unexpected. Fortunately this group of scientists realized that this is previously unknown molecule of pure carbon
After they obtained the Nobel Prize in Chemistry each of the three geniuses took a different path. Kroto used his fame to promote the scientific education. He became a critic of religious faith and a famous supporter of the British Humanist Association.
Richard Smalley was devoted to the nanotechnology for the rest of his life. He was also an advocate of the need for cheap, clean energy as he believed this would be the number one problem that humanity would face in 21st century.
Once he said: “Clean water is a great example of something that depends on energy. And if you solve the water problem, you solve the food problem.”
Unlike his colleagues, Robert Curl chose to remain out of the spotlights. He simply continued in his research. He explained it this way: “After winning a Nobel, you can either become a scientific pontificator, or you can have some idea for a new science project and you can use your newfound notoriety to get the resources to do it. Or you can say, 'Well, I enjoy what I was doing, and I want to keep doing that.”
And he kept doing it until 2008, when he retired at the age of 74.
C60 Fullerene – Meaning and Facts
The definition of formula C60 is clear now, but why it is also called Buckminsterfullerene or buckyballs? The molecule got its name after famous American architect, author, system theorist, futurist and inventor Richard Buckminster “Bucky” Fuller.
He developed an architectural design the geodesic dome. The structure of fullerenes is very similar to the one of the geodesic dome so the scientists decided to name the first fullerene after its architect.
However, it might be a bit misleading as the geodesic dome is not made of hexagons and pentagons. In fact it is built out of triangles.
So the name Buckminsterfullerene refers to the shape and structure and buckyball is just a shortened version of it.
The Secret of C60 Molecule Lies within Its Unique Structure
Let’s have a closer look at it. Buckyball is a truncated icosahedron which is a polyhedron with twenty faces. As it was stated in the beginning it consists of twenty hexagons and twelve pentagons.
Some icosahedrons are more symmetrical than the other, but our molecule C60 is actually the most symmetrical molecule of all.
It could be described as absolute perfection. In practice it means there are 120 operations, rotations around axis and reflections in the plane.
The symmetry of our molecule C60 is its most astonishing property.
Did You Know?
If you take buckyball molecule and compare it to an actual soccer ball, it will give you the same ratio as if you compare the soccer ball to the Earth.
More Interesting Facts You Also Need to Know
- C60 is the smallest buckyball. There are no pentagons in contact. It means that they do not share an edge or a corner with another pentagon.
- It was not any advanced technology that helped to determine the structure of buckyball. It is true that they tried to model its structure on the computer first, but it did not work out. So basically what happened was that Smalley took scissors, tape and paper and built the model by his own hands. First it did not work either as he used only hexagons, but once he listened to Harry Kroto and added pentagons as well, it closed. And that is how the first model of C60 was built.
- Regarding the structure and also the naming of Buckminsterfullerene, there was a small argument between Smalley and Kroto. Both of these two masterminds just could not remember who came up with the idea first. Robert Curl stayed out of this dispute claiming: “Harry was convinced that it was his idea and Rick was convinced it was his idea and I'm convinced it wasn't my idea.”
It is its outstanding structure that fascinates the scientists all around the world as it suggests many possible applications and that is also the reason why it is a subject of numerous researches and experiments.
Physical and Chemical Properties of C60 Buckyball
The Buckminsterfullerene is not only the most symmetrical molecule in the world. It has other extraordinary physical and chemical properties such as:
- Stability – this molecule is very stable and incredibly strong, which means it can resist high temperature and pressure.
- Solubility – it is not soluble in water, but it can be dissolved in aromatic solvents.
- Wave-particle duality.
- It behaves as it is electron deficient. This allows it to react with electron rich species
In addition C60 was also found in outer space.
Thanks to these exceptional properties the applications and uses of these molecules may be limitless.
Medical Applications
There are extensive researches in progress ever since the fullerenes were discovered. Its potential in biomedicine is huge and fullerenes give hope to millions of patients. How they could be used?
- Antioxidants – fullerenes are really effective anti-radicals shields. In fact one molecule of C60 can neutralized up to 34 methyl radicals. That is why it is also called the ‘radical sponge’. Thanks to these abilities fullerenes are used in cosmetology. It prevents skin damage and has anti-aging effect on the skin without any side effects. They could be also used as derivate of vitamin C and E.
- Antiviral agents – fullerenes have the ability to prevent the development of the human immunodeficiency virus (HIV), therefore it could postpone the outbreak of acquired immunodeficiency syndrome (AIDS). They can also inhibit hepatitis C virus.
- Drug and gene delivery – fullerenes are able to deliver the pharmaceutical compound exactly where it is needed. In other words to the site of an action. When it comes to gene delivery there are implemented foreign DNA into the cells in order to achieve a desired effect. In both cases it is very important to deliver these molecules safely and effectively. This way the treatment would be not only more effective, but it would also have less negative side-effects.
- Photodynamic Therapy and photosensitizers – Photodynamic Therapy (PDT) is a kind of a therapy that uses non-toxic light sensitive compound. When they are exposed to light, they become toxic. This capability is used to target malignant cells.
- Other biomedical applications could be in X-ray imagining or Magnetic Resonance Imagining.
Other Applications
- Hydrogen gas storage – thanks to their structure fullerenes can hydrogenate and dehydrogenate easily.
- Electrocatalysts – this could be used for example in the fuel cells. It could reduce the air pollution caused by burning the fossil fuels.
- Hardening agents – they could be used to develop lighter, but very strong metals.
- Composite coatings – fullerenes improve the quality of the common used coating agents
There is definitely a bright future ahead. Their importance and potential are recognized not only by experts, but also by individuals. Fullerenes are already successfully implemented into our lives. At this time the best practical use of them is in cosmetology, it is also very popular component of thermal pastes which protect the electronics.
It is expected there will be developed cost-effective method of synthesis soon. Then there would be available fullerenes for sale for lower prices.
Introducing Other Types of Fullerenes
Since 1985 the structural variations of fullerenes have developed. And as it was stated before we will have a closer look at the particular types as well.
- Buckyballs clusters – C60 belongs to this group. It is in fact the most common fullerene from the fullerene family.
- Nanotubes – they could be described as hollow tubes of really small dimensions. They can have single or multiple walls. Their possible applications are in electronics, cosmetology, composite and polymeric materials, lubricants, automotive industries or research and development.
- Megatubes – as its name suggests they are larger in size than nanotubes. Their walls have different thickness. Their possible application could be a transport of molecule of various dimensions.
- Polymers – There is used high pressure and temperature to produce two-dimensional and three-dimensional polymers.
- Nano “onions” – they could be used in lubricants.
- Linked “ball-and-chain” dimers – they are two buckyballs connected by a carbon chain.
- Fullerene rings.
The Definition of Fullerenes Is Clear. Its Full Potential Is Still a Mystery
Nanotechnology is a fast-evolving science. There are many ongoing researches in progress. For example our molecule C60 is one of the most tested nanomaterials.
It is a thrilling adventure. By its end we might have solutions for the most emerging problems such as energy deficiency, incurable diseases, pollution and the list could go on.
Carbon 60 Fullerene – The Summary
Now you can not only differentiate the types of fullerenes, but you also know all basic information about the first discovered fullerene, molecule C60.
You can recognize it by its shape and structure. You also know interesting facts about its discovery.
Last but not least you have a better idea about its huge potential that could be used in many applications.
Are fullerenes going to change our lives to the better? Is it a salvation for the mankind or the doom? Share your opinions on this topic with us.